








 Introduction
Three families have been found to specifically infect insects - Baculoviridae, Polydnaviridae and Ascoviridae
(University of Warwick, accessed online 2011). Viral entomopathogens however only account for 1% of the global
market for microbial biopesticides due to several limitations (Harper, 2006). Viruses are intracellular organisms so
they are difficult and expensive to grow in the laboratory. In addition, commercial use is restricted because they
are slow acting, sensitive to UV light and have a narrow host range, often for individual species (Lacey et al, 2001).
Once established in a population however, viruses are extremely effective as they are persistent, highly virulent
and produce secondary inoculum. They are safe to use as they do not infect vertebrates. Another advantage is that
resistance is extremely rare because the virus is living and therefore is able to evolve with its host. Viral pathogens
are typically used for inundative control.
Baculoviridae
The family Baculoviridae have the greatest potential for use in biological control of insects because they are very
specific and highly effective (Copping and Menn, 2000). Sixteen products based on baculoviruses are currently
available for use (University of Warwick, accessed online 2011). There are two genera within this family,
Nucleopolyhedrovirus (NPV) and Granulovirus (GV). NPV targets Lepidoptera and Hymenoptera. Helicoverpazea
NPV for example controls tobacco budworm and bollworms (Copping and Menn, 2000). Whereas, GV only targets
Lepidoptera. Cydia pomonella granulosis virus for example controls the codling moth, a universal, major pest of
apple, plums and pear trees, and is used on 15% of Washington State orchards (Copping and Menn, 2000). Little
research has focused on other potential viruses.
Viral Structure
The infectious agent of a baculovirus is called the occlusion body (OB) (Figure 1) (Harper, 2006). An OB refers to
the specific protein (polyhedrin in NPVs and granulin in GVs) that forms a thick layer that coats the
deoxyribonucleic acid (DNA) to form a single nucleocapsid (GV and NPV) or multiple nucleocapsids (NPV only). OBs
offer protection and give better environmental stability. Not all baculoviruses however have an OB.
Introduction
Three families have been found to specifically infect insects - Baculoviridae, Polydnaviridae and Ascoviridae
(University of Warwick, accessed online 2011). Viral entomopathogens however only account for 1% of the global
market for microbial biopesticides due to several limitations (Harper, 2006). Viruses are intracellular organisms so
they are difficult and expensive to grow in the laboratory. In addition, commercial use is restricted because they
are slow acting, sensitive to UV light and have a narrow host range, often for individual species (Lacey et al, 2001).
Once established in a population however, viruses are extremely effective as they are persistent, highly virulent
and produce secondary inoculum. They are safe to use as they do not infect vertebrates. Another advantage is that
resistance is extremely rare because the virus is living and therefore is able to evolve with its host. Viral pathogens
are typically used for inundative control.
Baculoviridae
The family Baculoviridae have the greatest potential for use in biological control of insects because they are very
specific and highly effective (Copping and Menn, 2000). Sixteen products based on baculoviruses are currently
available for use (University of Warwick, accessed online 2011). There are two genera within this family,
Nucleopolyhedrovirus (NPV) and Granulovirus (GV). NPV targets Lepidoptera and Hymenoptera. Helicoverpazea
NPV for example controls tobacco budworm and bollworms (Copping and Menn, 2000). Whereas, GV only targets
Lepidoptera. Cydia pomonella granulosis virus for example controls the codling moth, a universal, major pest of
apple, plums and pear trees, and is used on 15% of Washington State orchards (Copping and Menn, 2000). Little
research has focused on other potential viruses.
Viral Structure
The infectious agent of a baculovirus is called the occlusion body (OB) (Figure 1) (Harper, 2006). An OB refers to
the specific protein (polyhedrin in NPVs and granulin in GVs) that forms a thick layer that coats the
deoxyribonucleic acid (DNA) to form a single nucleocapsid (GV and NPV) or multiple nucleocapsids (NPV only). OBs
offer protection and give better environmental stability. Not all baculoviruses however have an OB.
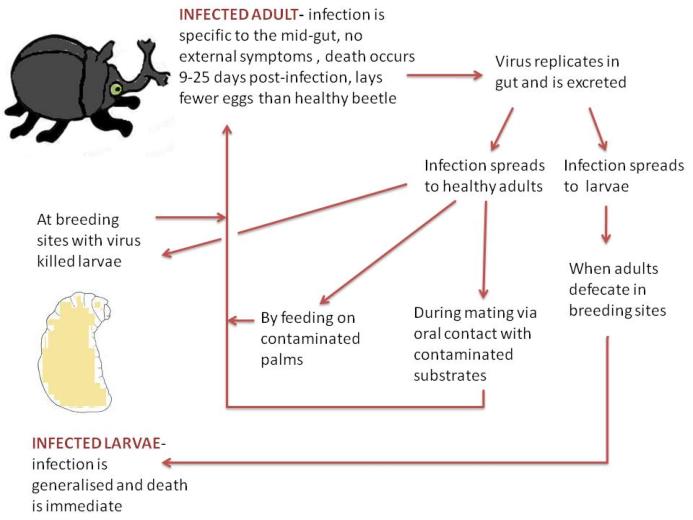 Autodissemination of the OrV (Lacey et al, 2001)
Future Prospects
In the future, exploitation of viruses is expected to increase as scientists develop ways to overcome their
limitations. In developing countries for example where cost of labour is low, virus production would provide
employment and be a cheaper option than importing pesticides. Genetic manipulation of some viruses will also
improve their efficacy, for example by shortening the lag time between time of ingestion and death.
Autodissemination of the OrV (Lacey et al, 2001)
Future Prospects
In the future, exploitation of viruses is expected to increase as scientists develop ways to overcome their
limitations. In developing countries for example where cost of labour is low, virus production would provide
employment and be a cheaper option than importing pesticides. Genetic manipulation of some viruses will also
improve their efficacy, for example by shortening the lag time between time of ingestion and death.
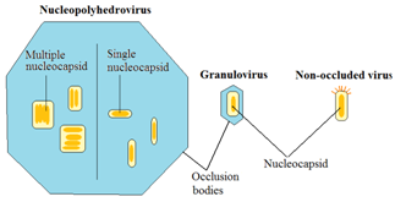 Figure 1 Baculovirus structure. Diagram adapted from Harper (2006).
Figure 1 Baculovirus structure. Diagram adapted from Harper (2006).


 Infection Process
Baculoviruses cause infection via ingestion by larvae. The occlusion bodies dissolve in the alkaline gut which frees
the virions. Virions then bind to gut receptors and invade the gut epithelial cells where it replicates and grows in
the nucleus. The virus then spreads across the body in the haemolymph and causes the larvae to die. In
Lepidoptera, death typically occurs within 4-5 days after infection. A new generation of virus is then released from
the dead body ready to invade a new host. Transmission of virus within the host insect populations can be both
horizontal and vertical.
Classical Control by the non-occluded baculovirus, Oryctes rhinoceros virus
Oryctes rhinoceros virus (OrV) infects the rhinoceros beetle (Oryctes rhinoceros), a serious pest in the tropics that
destroys coconut palms and which cannot be controlled with pesticides (Huger, 2005). In 1968 alone the beetle
caused serious crop damage in South Pacific countries, with an estimated loss of $US 1,100,000 (Bedford, 1980).
The adults are responsible for the damage as they eat the palm crown, which lowers yield and kills saplings. Old
rotting palm logs provide breeding grounds for the beetle and are where the larvae develop. The virus was
discovered in Malaysia and has been used to inoculate O. rhinoceros populations below their economic thresholds
since 1967 (Huger, 2005). OrV can persist at low host densities which gives it successful long term control. To
maintain permanent and optimal control however, plantation hygiene such as removing breeding sites is
important. The adult stage is a very active vector for disseminating the virus and spread can be enhanced by
applying the virus to the mouth parts of captured beetles.
Infection Process
Baculoviruses cause infection via ingestion by larvae. The occlusion bodies dissolve in the alkaline gut which frees
the virions. Virions then bind to gut receptors and invade the gut epithelial cells where it replicates and grows in
the nucleus. The virus then spreads across the body in the haemolymph and causes the larvae to die. In
Lepidoptera, death typically occurs within 4-5 days after infection. A new generation of virus is then released from
the dead body ready to invade a new host. Transmission of virus within the host insect populations can be both
horizontal and vertical.
Classical Control by the non-occluded baculovirus, Oryctes rhinoceros virus
Oryctes rhinoceros virus (OrV) infects the rhinoceros beetle (Oryctes rhinoceros), a serious pest in the tropics that
destroys coconut palms and which cannot be controlled with pesticides (Huger, 2005). In 1968 alone the beetle
caused serious crop damage in South Pacific countries, with an estimated loss of $US 1,100,000 (Bedford, 1980).
The adults are responsible for the damage as they eat the palm crown, which lowers yield and kills saplings. Old
rotting palm logs provide breeding grounds for the beetle and are where the larvae develop. The virus was
discovered in Malaysia and has been used to inoculate O. rhinoceros populations below their economic thresholds
since 1967 (Huger, 2005). OrV can persist at low host densities which gives it successful long term control. To
maintain permanent and optimal control however, plantation hygiene such as removing breeding sites is
important. The adult stage is a very active vector for disseminating the virus and spread can be enhanced by
applying the virus to the mouth parts of captured beetles.









 Introduction
Three families have been found to specifically infect insects - Baculoviridae, Polydnaviridae and Ascoviridae
(University of Warwick, accessed online 2011). Viral entomopathogens however only account for 1% of the global
market for microbial biopesticides due to several limitations (Harper, 2006). Viruses are intracellular organisms so
they are difficult and expensive to grow in the laboratory. In addition, commercial use is restricted because they
are slow acting, sensitive to UV light and have a narrow host range, often for individual species (Lacey et al, 2001).
Once established in a population however, viruses are extremely effective as they are persistent, highly virulent
and produce secondary inoculum. They are safe to use as they do not infect vertebrates. Another advantage is that
resistance is extremely rare because the virus is living and therefore is able to evolve with its host. Viral pathogens
are typically used for inundative control.
Baculoviridae
The family Baculoviridae have the greatest potential for use in biological control of insects because they are very
specific and highly effective (Copping and Menn, 2000). Sixteen products based on baculoviruses are currently
available for use (University of Warwick, accessed online 2011). There are two genera within this family,
Nucleopolyhedrovirus (NPV) and Granulovirus (GV). NPV targets Lepidoptera and Hymenoptera. Helicoverpazea
NPV for example controls tobacco budworm and bollworms (Copping and Menn, 2000). Whereas, GV only targets
Lepidoptera. Cydia pomonella granulosis virus for example controls the codling moth, a universal, major pest of
apple, plums and pear trees, and is used on 15% of Washington State orchards (Copping and Menn, 2000). Little
research has focused on other potential viruses.
Viral Structure
The infectious agent of a baculovirus is called the occlusion body (OB) (Figure 1) (Harper, 2006). An OB refers to
the specific protein (polyhedrin in NPVs and granulin in GVs) that forms a thick layer that coats the
deoxyribonucleic acid (DNA) to form a single nucleocapsid (GV and NPV) or multiple nucleocapsids (NPV only). OBs
offer protection and give better environmental stability. Not all baculoviruses however have an OB.
Introduction
Three families have been found to specifically infect insects - Baculoviridae, Polydnaviridae and Ascoviridae
(University of Warwick, accessed online 2011). Viral entomopathogens however only account for 1% of the global
market for microbial biopesticides due to several limitations (Harper, 2006). Viruses are intracellular organisms so
they are difficult and expensive to grow in the laboratory. In addition, commercial use is restricted because they
are slow acting, sensitive to UV light and have a narrow host range, often for individual species (Lacey et al, 2001).
Once established in a population however, viruses are extremely effective as they are persistent, highly virulent
and produce secondary inoculum. They are safe to use as they do not infect vertebrates. Another advantage is that
resistance is extremely rare because the virus is living and therefore is able to evolve with its host. Viral pathogens
are typically used for inundative control.
Baculoviridae
The family Baculoviridae have the greatest potential for use in biological control of insects because they are very
specific and highly effective (Copping and Menn, 2000). Sixteen products based on baculoviruses are currently
available for use (University of Warwick, accessed online 2011). There are two genera within this family,
Nucleopolyhedrovirus (NPV) and Granulovirus (GV). NPV targets Lepidoptera and Hymenoptera. Helicoverpazea
NPV for example controls tobacco budworm and bollworms (Copping and Menn, 2000). Whereas, GV only targets
Lepidoptera. Cydia pomonella granulosis virus for example controls the codling moth, a universal, major pest of
apple, plums and pear trees, and is used on 15% of Washington State orchards (Copping and Menn, 2000). Little
research has focused on other potential viruses.
Viral Structure
The infectious agent of a baculovirus is called the occlusion body (OB) (Figure 1) (Harper, 2006). An OB refers to
the specific protein (polyhedrin in NPVs and granulin in GVs) that forms a thick layer that coats the
deoxyribonucleic acid (DNA) to form a single nucleocapsid (GV and NPV) or multiple nucleocapsids (NPV only). OBs
offer protection and give better environmental stability. Not all baculoviruses however have an OB.
 Autodissemination of the OrV (Lacey et al, 2001)
Future Prospects
In the future, exploitation of viruses is expected to increase as scientists develop ways to overcome their
limitations. In developing countries for example where cost of labour is low, virus production would provide
employment and be a cheaper option than importing pesticides. Genetic manipulation of some viruses will also
improve their efficacy, for example by shortening the lag time between time of ingestion and death.
Autodissemination of the OrV (Lacey et al, 2001)
Future Prospects
In the future, exploitation of viruses is expected to increase as scientists develop ways to overcome their
limitations. In developing countries for example where cost of labour is low, virus production would provide
employment and be a cheaper option than importing pesticides. Genetic manipulation of some viruses will also
improve their efficacy, for example by shortening the lag time between time of ingestion and death.
 Figure 1 Baculovirus structure. Diagram adapted from Harper (2006).
Figure 1 Baculovirus structure. Diagram adapted from Harper (2006).


 Infection Process
Baculoviruses cause infection via ingestion by larvae. The occlusion bodies dissolve in the alkaline gut which frees
the virions. Virions then bind to gut receptors and invade the gut epithelial cells where it replicates and grows in
the nucleus. The virus then spreads across the body in the haemolymph and causes the larvae to die. In
Lepidoptera, death typically occurs within 4-5 days after infection. A new generation of virus is then released from
the dead body ready to invade a new host. Transmission of virus within the host insect populations can be both
horizontal and vertical.
Classical Control by the non-occluded baculovirus, Oryctes rhinoceros virus
Oryctes rhinoceros virus (OrV) infects the rhinoceros beetle (Oryctes rhinoceros), a serious pest in the tropics that
destroys coconut palms and which cannot be controlled with pesticides (Huger, 2005). In 1968 alone the beetle
caused serious crop damage in South Pacific countries, with an estimated loss of $US 1,100,000 (Bedford, 1980).
The adults are responsible for the damage as they eat the palm crown, which lowers yield and kills saplings. Old
rotting palm logs provide breeding grounds for the beetle and are where the larvae develop. The virus was
discovered in Malaysia and has been used to inoculate O. rhinoceros populations below their economic thresholds
since 1967 (Huger, 2005). OrV can persist at low host densities which gives it successful long term control. To
maintain permanent and optimal control however, plantation hygiene such as removing breeding sites is
important. The adult stage is a very active vector for disseminating the virus and spread can be enhanced by
applying the virus to the mouth parts of captured beetles.
Infection Process
Baculoviruses cause infection via ingestion by larvae. The occlusion bodies dissolve in the alkaline gut which frees
the virions. Virions then bind to gut receptors and invade the gut epithelial cells where it replicates and grows in
the nucleus. The virus then spreads across the body in the haemolymph and causes the larvae to die. In
Lepidoptera, death typically occurs within 4-5 days after infection. A new generation of virus is then released from
the dead body ready to invade a new host. Transmission of virus within the host insect populations can be both
horizontal and vertical.
Classical Control by the non-occluded baculovirus, Oryctes rhinoceros virus
Oryctes rhinoceros virus (OrV) infects the rhinoceros beetle (Oryctes rhinoceros), a serious pest in the tropics that
destroys coconut palms and which cannot be controlled with pesticides (Huger, 2005). In 1968 alone the beetle
caused serious crop damage in South Pacific countries, with an estimated loss of $US 1,100,000 (Bedford, 1980).
The adults are responsible for the damage as they eat the palm crown, which lowers yield and kills saplings. Old
rotting palm logs provide breeding grounds for the beetle and are where the larvae develop. The virus was
discovered in Malaysia and has been used to inoculate O. rhinoceros populations below their economic thresholds
since 1967 (Huger, 2005). OrV can persist at low host densities which gives it successful long term control. To
maintain permanent and optimal control however, plantation hygiene such as removing breeding sites is
important. The adult stage is a very active vector for disseminating the virus and spread can be enhanced by
applying the virus to the mouth parts of captured beetles.